RADIOMETRIC DATING
I. DEFINITIONS:
element: The smallest division of matter that still retains its
chemical properties. The chemical properties are determined by the number
of electrons in the neutral atom which in turn is determined by the number
of protons in the nucleus of the atom.
isotope: An isotope of an element has the same number of protons
but a different number of neutrons in its nucleus and therefore differs
in atomic mass but has the same chemical properties.
radioactive atom: An unstable isotope that spontaneously changes
into another form by emitting a particle from its nucleus. The other form
will be an isotope of a new element with a different number of protons.
parent isotope: The term for the original radioactive atoms which
decay at a known rate. In some cases the parent isotopes decay through
a series of radioactive isotopes before reaching a stable isotope.
daughter (radiogenic) isotope: The term for the isotopes that
result from the decay of the parent isotopes. For the purpose of radiometric
dating of rocks, it is the final stable daughter isotope that is of interest.
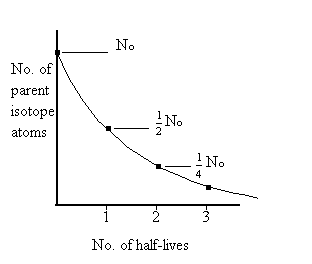 half-life:
The time required for half the original parent isotope to decay into its
daughter isotope. For example: If the half-life of the parent isotope was
500 years and the original number of atoms was 1 billion, after 1 half-life
(500 years) there would be 500 million (1/2 billion) atoms of the original
parent isotope left. After another half-life (1000 years = 2 half-lives)
there would be 1/2 of the 1/2 billion atoms left, or 1/4 billion (250 million)
atoms. See figure.
half-life:
The time required for half the original parent isotope to decay into its
daughter isotope. For example: If the half-life of the parent isotope was
500 years and the original number of atoms was 1 billion, after 1 half-life
(500 years) there would be 500 million (1/2 billion) atoms of the original
parent isotope left. After another half-life (1000 years = 2 half-lives)
there would be 1/2 of the 1/2 billion atoms left, or 1/4 billion (250 million)
atoms. See figure.
II. RADIOMETRIC DATING PROCEDURE FOR ROCKS:
Many minerals contain radioactive atoms which can be used to determine
the age of the mineral and therefore the age of the rock containing the
mineral. The age that is measured is the time since the rock began to retain
the daughter isotope. This is usually the time since the rock solidified
so that the daughter isotope atoms could be trapped in the solid mineral
structure. Since sedimentary rocks are composed of previously formed igneous
and metamorphic rocks and minerals, their formation ages cannot be determined
by most of the radiometric dating techniques. Igneous rocks are the best
candidates for these techniques and metamorphic rocks can be dated to their
last episode of metamorphism.
In order to date the formation age of a rock, the original number
of parent and daughter isotope atoms must be determined. It is also necessary
to assume that there has been no addition or loss of either parent or daughter
isotope since the minerals solidified. In most cases, it is actually the
rate of decay (activity) that is measured rather than the total number
of atoms. The activity is proportional to the total number of radioactive
atoms in the sample. In some cases the original ratio of parent to daughter
isotope can be measured by comparing different mineral grains in the same
rock. In some cases the original number of parent isotope atoms can be
determined by measuring the numbers of stable daughter isotope atoms which
usually occur in certain proportions to the unstable parent isotope atoms
in a given fresh material. (eg: A recently solidified lava rock with the
same minerals.) Then by counting the present number of parent and
daughter isotope atoms in the mineral, we can determine how many parent
atoms have decayed into daughter atoms and hence determine the time since
the rock solidified. For example, if it is determined that one half the
original parent isotopes have decayed, then the rock is one half-life old.
The formula for determining the age of a mineral given T½
(half-life of the parent isotope), N (present number of parent isotopes),
and No (original number of parent isotopes) follows:
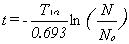
Some of the parent isotopes most widely used to date rocks are:
|
Parent
|
Stable Daughter
|
Half-Life
|
|
Uranium-235
|
Lead-207
|
0.713 billion years
|
|
Uranium-238
|
Lead-206
|
4.51 billion years
|
|
Rubidium-87
|
Strontium-87
|
47 billion years
|
 half-life:
The time required for half the original parent isotope to decay into its
daughter isotope. For example: If the half-life of the parent isotope was
500 years and the original number of atoms was 1 billion, after 1 half-life
(500 years) there would be 500 million (1/2 billion) atoms of the original
parent isotope left. After another half-life (1000 years = 2 half-lives)
there would be 1/2 of the 1/2 billion atoms left, or 1/4 billion (250 million)
atoms. See figure.
half-life:
The time required for half the original parent isotope to decay into its
daughter isotope. For example: If the half-life of the parent isotope was
500 years and the original number of atoms was 1 billion, after 1 half-life
(500 years) there would be 500 million (1/2 billion) atoms of the original
parent isotope left. After another half-life (1000 years = 2 half-lives)
there would be 1/2 of the 1/2 billion atoms left, or 1/4 billion (250 million)
atoms. See figure.